Research
Single-molecule Studies of Telomere Maintenance

Telomeres play important roles in maintaining the stability of linear chromosomes. The telomeric structure allows a cell to distinguish between natural chromosome ends and double-stranded DNA breaks. Telomere dysfunction and associated chromosomal abnormalities have been strongly associated with age-associated degenerative diseases and cancer. The mechanisms driving telomere maintenance that involve dynamic actions of multiple proteins on a long and complex DNA structure are not well understood. Using single-molecule fluorescence imaging of quantum dot (QD)-labeled TRF1 and TRF2, we discovered how TRF1 and TRF2 use different mechanisms to find telomeric DNA but share a novel mechanism to search for protein partners at telomeres. We demonstrated that TIN2S and TIN2L isoforms facilitate TRF1-mediated DNA compaction (cis-interactions) and DNA-DNA bridging (trans-interactions) in a telomeric sequence- and length-dependent manner. In addition, TRF1 tethers cohesin SA1 within telomeric regions that SA1 transiently interacts with. SA1 and TRF1 together form longer DNA-DNA pairing tracts than with TRF1 alone. These results suggest that at telomeres, cohesion relies on the molecular interplay between TRF1 and SA1 to promote DNA-DNA pairing.
Single-molecule Studies of the Cohesin complex

The cohesin complex plays important roles in sister chromatid cohesion, DNA replication, repair, and recombination, as well as 3D chromosome organization. In vertebrates, the core cohesin complex consists of a tripartite ring assembled from SMC1, SMC3, and RAD21 (also known as SCC1), and the stromal antigen subunit (SA) SA1 (STAG1) or SA2 (STAG2). The biophysical properties and activities of the cohesin SA1 and SA2 are largely unknown. We established that SA1 and SA2 bind to both dsDNA and ssDNA. Our results suggest that SA2 functions at intermediate DNA structures during DNA transactions in genome maintenance pathways. We discovered that both SA1 and SA2 bind to various RNA-containing substrates, including ssRNA, dsDNA, RNA:DNA hybrids, and R-loops. This discovery of previously unknown DNA and RNA binding activities of cohesin SA1 and SA2 opens up new directions of research to unravel the mechanisms underlying their diverse cellular functions.
AFM and Dual-Resonance frequency- Enhanced EFM (DREEM)

AFM generates an image of a surface by scanning with a sharp sensor tip attached to a cantilever. The most commonly applied AFM imaging mode is the intermittent contact (or oscillating) mode. In addition, we apply Dual-Resonance-frequency-Enhanced Electrostatic force microscopy (DREEM) to resolve ds and ssDNA within protein-DNA complexes and High-speed AFM imaging in liquids to uncover protein-DNA binding dynamics.
DNA Tightrope
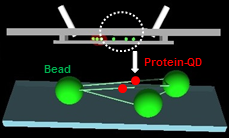
This assay enables us to directly observe movements of individual proteins with up to 16 nm positional accuracy and 50 ms temporal resolution.
Microscopes

AFM and DREEM
The MFP-3D-BIO Atomic Force Microscope (AFM) from Asylum Research is a high performance AFM specifically designed for biological applications. This microscope is equipped with a BioHeater, an Environmental Controller, and an iDrive Kit for visualizing protein-DNA samples in solution. Scan Size: X&Y axes: 90um range in closed loop; Resolution: 0.25 nm. It can carry “autoscan” to automatically scan a 90 um by 90 um area. A Stanford Research SR844RF locking amplifier and 3.1MHz synthesized function generator are used for the new DREEM imaging technique.

Fluorescence Microscope
This Nikon microscope is equipped with an encoded motorized stage, perfect focus system (PFS), a Ti-TIRF E motorized illuminator unit, a 488 nm solid state laser, a 100X TIRF objective, a Dual View device, and an electron multiplied (EM) CCD camera (iXon DU897, Andor Technology). The excitation beam is reflected into the objective through a TIRF filter set containing zt488rdc and ET500LP filters. For simultaneous imaging of green (565 nm) and red (655 nm) Qdots, a dual view simultaneous imaging system was used in combination with a T605LPXR dichroic beam splitter and a bandpass filter ET655/40m (Chroma).